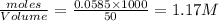The given question is incomplete. The complete question is
Calculate the mass of AgCl formed, and the concentration of silver ion remaining in solution, when 10.0g of solid
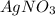 is added to 50.mL of
is added to 50.mL of
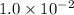 NaCl. Assume there is no volume change upon addition of the solid.
NaCl. Assume there is no volume change upon addition of the solid.
Answer: a) mass of AgCl formed = 0.072 g
b) Concentration of silver ion remaining in solution
Step-by-step explanation:
To calculate the moles :
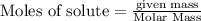
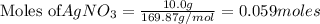
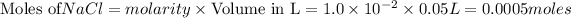
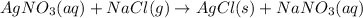
According to stoichiometry :
1 mole of
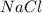 require = 1 mole of
require = 1 mole of
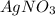
Thus 0.0005 moles of
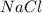 will require=
will require=
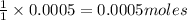 of
of
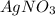
Thus
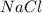 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
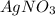 is the excess reagent.
is the excess reagent.
As 1 mole of NaCl give = 1 mole of AgCl
Thus 0.0005 moles of NaCl give =
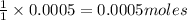 of
of

Mass of
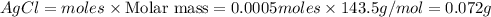
moles of AgCl left = (0.059-0.0005) = 0.0585
Concentration of
![[Ag]^+](https://img.qammunity.org/2021/formulas/chemistry/high-school/ie1qzojg94vt5u7q4hh39q5zsiviy1wmb8.png) left in solution =
left in solution =