The given question is incomplete. The complete question is :
Calculate to three significant digits the density of boron trifluoride gas at exactly −5°C and exactly 1 atm . You can assume boron trifluoride gas behaves as an ideal gas under these conditions.
Answer: The density of boron trifluoride gas is 3.08 g/L
Step-by-step explanation:
To calculate the relation of density and molar mass of a compound, we use the ideal gas equation:
PV=nRT
P = pressure = 1 atm
V = Volume
n = number of moles
R = gas constant =0.0821 Latm/Kmol
T = temperature =

Number of moles (n) can be written as:
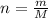
where, m = given mass
M = molar mass
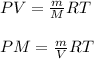
where,
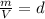
where d = density
The relation becomes:
PM=dRT


Thus the density of boron trifluoride gas is 3.08 g/L