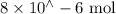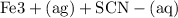 <---->
<---->
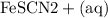
Step-by-step explanation:
Use the concentration and volume values they've given you to find the moles of Fe(NO3)3 and KSCN that were initially present (before they were mixed)
moles = concentration in M x volume in L
n(Fe(NO3)3) =
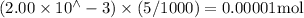
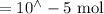
n(KSCN) =
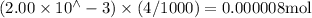
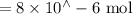
n(Fe(NO3)3) = n(Fe3+) =
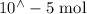
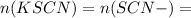
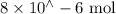
so n(Fe3+) initially present =
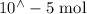
n(SCN-) initially present =