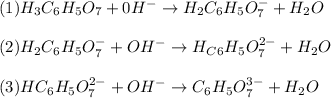Answer:
Net ionic equation are as follows
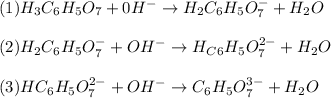
Step-by-step explanation:
Citric acid, H3C6H5O7, is a triprotic acid
he net ionic equation for the reaction that occurs when NaOH is added to a buffer containing H2C6H5O7 - and HC6H5O7 2-
Net ionic equation are as follows