The given question is incomplete. The complete question is
The thermite reaction, in which powdered aluminum reacts with iron oxide, is highly exothermic:
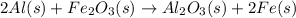 . Use standard enthalpies of formation to find ΔH∘rxn for the thermite reaction. Express the heat of the reaction in kilojoules to four significant figures.
. Use standard enthalpies of formation to find ΔH∘rxn for the thermite reaction. Express the heat of the reaction in kilojoules to four significant figures.
Answer: ΔH∘rxn for the thermite reaction is -851.5 kJ
Step-by-step explanation:
The balanced chemical reaction is :
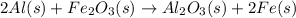
We have to calculate the enthalpy of reaction
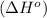 .
.
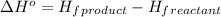
![\Delta H^o=[n_(Fe)* \Delta H_f^0_((Fe))+n_(Al_2O_3)* \Delta H_f^0_((Al_2O_3))]-[n_(Al)* \Delta H_f^0_(Al)+n_(Fe_2O_3)* \Delta H_f^0_((Fe_2O_3))]](https://img.qammunity.org/2021/formulas/chemistry/college/qod6mixqsei03tb7kckwkrh98rbaefbmuq.png)
where,
We are given:
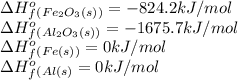
Putting values in above equation, we get:
![\Delta H^o_(rxn)=[(2* 0)+(1* -1675.5)]-[(2* 0)+(1* -824.2)]=-851.5kJ](https://img.qammunity.org/2021/formulas/chemistry/college/5pf92mlyefd26b8gxcb886pavub5kzn0cv.png)
ΔH∘rxn for the thermite reaction is -851.5 kJ