Answer:
Pressure=3.91atm
Step-by-step explanation:
As given in the question that gas is ideal so
we can use Ideal gas equation

volume of gas is not given directly but volume of container is given and we know gas takes the volume in which they are kept.
therefore mole remains contant
P1=2.5 atm
V1=2L
T1=320K
P1=?
V1=1L
T1=250K
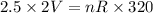 ..............(1)
..............(1)
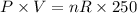 ................(2)
................(2)
eqn2/eqn1
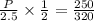
Pressure=3.91atm