Answer:
The moles of starting material is
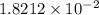 mole
mole
Step-by-step explanation:
Given:
Cyclohexene oxide material is used
Density = 0.97
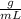
Volume = 1.84 mL
Formula of Cyclohexene oxide =
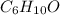
Molar mass is given by,
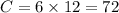
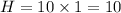
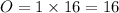
⇒
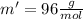
Mass
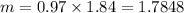
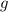
Moles is given by,
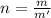
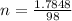
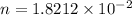 mol
mol
Therefore, the moles of starting material is
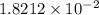 mole
mole