Answer:
a)The percentage yield of the reaction is 87.3%.
b) 0.343 gram is the mass of CO that passed through.
Step-by-step explanation:
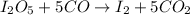
Moles of carbon monoxide gas =
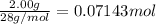
According to reaction, 5 moles of carbon monoxide gives 1 moles of iodine gas,then 0.07143 moles of carbon monoxide will give :
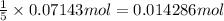 of iodine gas
of iodine gas
Mass of 0.014286 moles of iodine gas:
0.014286 mol × 254 g /mol = 3.63 g
Theoretical yield of the iodine gas = 3.63 g
Experimental yield of the iodine gas = 3.17 g
Percentage yield of the reaction :
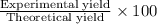
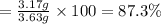
The percentage yield of the reaction is 87.3%.
b)
Mass of iodine gas produced = 3.17 g
Moles of iodine gas =
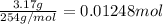
According to reaction , 1 mole of iodine gas is obtained from 5 moles of carbon monoxide gas, then 0.01248 moles of iodine gas will be obtained from :
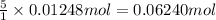 of carbon monoxide
of carbon monoxide
Moles of carbon monoxide reacted = 0.06240 mol
Moles of carbon monoxide gas used = 0.07143 mol
Moles of carbon monoxide gas which do not reacted:
0.07143 mol - 0.06240 mol = 0.00903 mol
Mass of 0.00903 moles of carbon monoxide gas:
0.00903 mol × 28 g/mol = 0.343 g
0.343 gram is the mass of CO that passed through.