Answer:
2.8g
Step-by-step explanation:
Given parameters:
volume of H₂O = 3.5L
Unknown:
Mass of water = ?
Solution:
One very important assumption to make is that this question is based on the standard temperature and pressure conditions.
Having this at the back of our minds makes this problem solvable.
For gases at STP;
Number of moles of water =
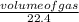
Solve for the number of moles;
Number of moles =
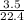 = 0.16mole
= 0.16mole
To solve for the mass;
Mass = number of moles x molar mass
Molar mass of H₂O = 2(1) + 16 = 18g/mol
Mass = 0.16 x 18 = 2.8g