Answer:
1. balanced equation for the reaction:
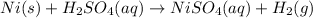
2. percentge of nickel=58.1%
Step-by-step explanation:
As given in the question that only nickel metal is present so hydrogen gas is produced by only nickel and there will be other substance too but only nickel can produced hydrogen as as reaction between metal and acid gives hydrogen gas.
1.
balanced equation for the reaction:
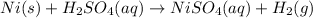
2.
From stoichiometry, we know
1 mole H_2 formed from 1 mole of Ni(s)
therefore 2 gram formed from 58.7 gram of Ni(s)
0.0764 gram formed from
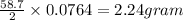
2.24 gram pure Ni(s) needs to produce 0.0764 gram of hydrogen
but this much hydrogen is produced from 3.86 gram of ore.
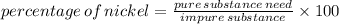
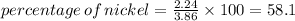
percentge of nickel=58.1%