Answer:
Approximately
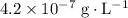 .
.
Step-by-step explanation:
Start by finding the concentration of
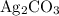 at equilibrium. The solubility equilibrium for
at equilibrium. The solubility equilibrium for
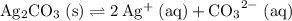 .
.
The ratio between the coefficient of
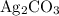 and that of
and that of
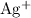 is
is
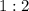 . For
. For
Let the increase in
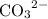 concentration be
concentration be
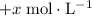 . The increase in
. The increase in
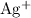 concentration would be
concentration would be
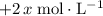 . Note, that because of the
. Note, that because of the
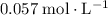 of
of
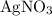 , the concentration of
, the concentration of
- The concentration of
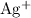 would be
would be
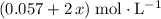 .
. - The concentration of
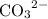 would be
would be
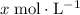 .
.
Apply the solubility product expression (again, note that in the equilibrium, the coefficient of
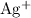 is two) to obtain:
is two) to obtain:
![\begin{aligned}&\rm \left[Ag^(+)\right]^2 \cdot \left[{CO_3}^(2-)\right] = K_{\text{sp}} \\ & \implies (0.057 + x)^2\cdot x = 8.1 * 10^(-12) \end{aligned}](https://img.qammunity.org/2021/formulas/chemistry/college/gwq3smtf6bb68pc7egjym12ruejimioifo.png) .
.
Note, that the solubility product of
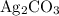 ,
,
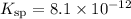 is considerably small. Therefore, at equilibrium, the concentration of
is considerably small. Therefore, at equilibrium, the concentration of
Apply this approximation to simplify
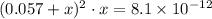 :
:
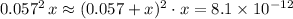 .
.
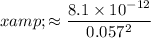 .
.
Calculate solubility (in grams per liter solution) from the concentration. The concentration of
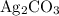 is approximately
is approximately
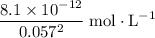 , meaning that there are approximately
, meaning that there are approximately
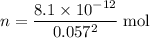 of
of
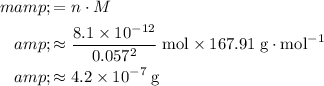 .
.
As a result, the maximum solubility of
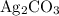 in this solution would be approximately
in this solution would be approximately
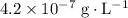 .
.