Answer:
Molar mass of solute: 300g/mol
Step-by-step explanation:
Vapor pressure of pure benzene: 0.930 atm
Assuming you dissolve 10.0 g of the non-volatile solute in 78.11g of benzene and vapour pressure of solution was found to be 0.900atm
It is possible to answer this question based on Raoult's law that states vapor pressure of an ideal solution is equal to mole fraction of the solvent multiplied to pressure of pure solvent:
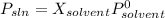
Moles in 78.11g of benzene are:
78.11g benzene × (1mol / 78.11g) = 1 mol benzene
Now, mole fraction replacing in Raoult's law is:
0.900atm / 0.930atm = 0.9677 = moles solvent / total moles.
As mole of solvent is 1:
0.9677× total moles = 1 mole benzene.
Total moles:
1.033 total moles. Moles of solute are:
1.033 moles - 1.000 moles = 0.0333 moles.
As molar mass is the mass of a substance in 1 mole. Molar mass of the solute is:
10.0g / 0.033moles = 300g/mol