Answer:
The reaction is an exothermic reaction .
The value of q is -880 kilo Joules.
Step-by-step explanation:
Exothermic reactions are defined as the reactions in which energy of reactants is more than the energy of the products. In these reactions, energy is released by the system. The total enthalpy of the reaction
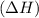 comes out to be negative.
comes out to be negative.
According to reaction, 2 moles of ammonia when reacts with 3 moles of nitrous oxide to give 4 moles of nitrogen gas and 3 moles of water vapor, along with release of 880 kJ of heat energy.
Since, heat is evolved during the course of reaction which means that reaction is an example of Exothermic reaction.
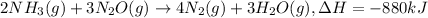
The reaction is an exothermic reaction .
The value of q is -880 kilo Joules.