Answer:
Heat released =811.68kcal
Step-by-step explanation:
2 CO(g) + O2(g) → 2 CO2(g) ΔH=-135.28kcal
for this type of question 1st balance the chemical reaction and use unitry method.
From the above balanced equaion it is clearly that,
2 mole of CO reacts with 1 mole of O2 and we have sufficient amount of oxygen means excess amount.
and also for complete consumption of 2 mole of CO, ΔH=-135.28 kcal
for complete consumption of 12 moles of CO2 ΔH=
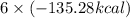
Heat released =811.68kcal