Answer: Glassware C
Step-by-step explanation:
According to moalrity equation:

 = Molarity of stock solution = 4.50 M
= Molarity of stock solution = 4.50 M
 = volume of stock solution = ?
= volume of stock solution = ?
 = molaity of diluted solution = 1.35 M
= molaity of diluted solution = 1.35 M
 = volume of diluted solution = 250.0 ml
= volume of diluted solution = 250.0 ml
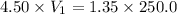
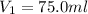
A volumetric flask is more accurate for making solutions than a beaker or erlenmeyer flask and hence to measure 75.0 ml of stock solution , we use 250 ml of volumetric flask.
Thus 75.0 ml of stock is pipetted out using pipette and put into a volumetric flask of 250 ml and water is added till the mark reaches 250 ml.
A volumetric flask is more accurate for making solutions than a beaker or erlenmeyer flask and hence to measure 75.0 ml of stock solution , we use 250 ml of volumetric flask.