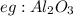Answer:
Correct option: 3
Step-by-step explanation:
A basic oxide is an oxide that reacts with water to give base or reacts with acid to form salt
eg:
Sodium oxide reacts with water to produce sodium hydroxide.
Magnesium oxide gives magnesium chloride on reaction with hydrochloric acid
An acidic oxide is an oxide that reacts with water to give acid and reacts with base to give salt.
eg: Carbon dioxide gives carbonic acid on reaction with water .
Sulfur dioxide gives sulfites when reacs reacts with bases.
An amphoteric oxide is an oxide which reacts with base as well acid and it shows because metal have multiple oxidation state and they can form oxide as well hydroxide