Answer:
176.1 g/mol ,
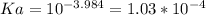
Step-by-step explanation:
The equation for the reaction is as shown in the attached diagram below
the reaction of Sodium Hydroxide, NaOH with ascorbic acid, C₆H₈O₆ is similar to the reaction of Potassium Hydroxide, KOH with ascorbic acid as show
C₆H₈O₆ + KOH ⇒ C₆H₇O₅K + H₂O
to produce one mole of potassium ascorbate and water
now,
0.1103 M KOH is contained in 1000mL
x moles is contained in 20mL
cross multiply making x the subject
No of moles of KOH = (0.1103 x 28.42)/1000 = 0.003135 moles
or
Moles KOH = 0.1103 x 0.02842 L = 0.003135 = moles ascorbic acid
Molar mass = 0.552 g / 0.003135 mol = 176.1 g/mol
Moles ascorbic acid = 0.552 / 176.1 =0.00313
moles NaOH = 0.0100 L x 0.1103 =0.001103
C₆H₈O₆ + OH⁻ >> C₆H₇O₆⁻ + H2O
Moles ascorbic acid in excess = 0.00313 - 0.001103 = 0.002027
Moles C₆H₇O₆⁻ = 0.001103
total volume = 20 + 10 = 30 mL = 0.030 L
concentration ascorbic acid = 0.002027 / 0.030 =0.0676 M
concentration C₆H₇O₆⁻ = 0.001103 / 0.030 =0.0368 M
pH = pKa + log [C₆H₇O₆⁻] / [C₆H₈O₆]
3.72 = pKa + log 0.0368 / 0.0676
3.72 = pKa - 0.264
pKa =3.984
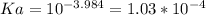
Ka = 10^-3.984 =1.03 x 10^-4