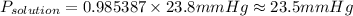Answer:
Step-by-step explanation:
As per Rault's law, when a solute is dissolved, the vapor pressure of the solution is equal to the product of the mole fraction of the solvent and the vapor pressure of the pure solvent.
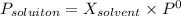
1. Calculate the mole fraction of solvent
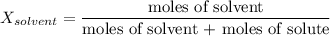
a) Moles fo solvent
The solven is water
- 3.28 liters of H₂O = 3,280 g of H₂O (taking density equal to 1.000 g/mol.
- number of moles = mass in grams/molar mass
- molar mass of H₂O = 18.015 g/mol
- number of moles of H₂O = 3,280g / (18.015g/mol) = 182.07 mol
b) Moles of solute
The solute is tang:
c) Mole fraction of solvent:
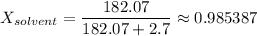
2. Vapor pressure of the solution