Answer:
2.25 M is the final concentration of hydroxide ions ions in the solution after the reaction has gone to completion.
Step-by-step explanation:
Moles of NaOH =
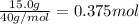
Molarity of the nitric acid solution = 0.250 M
Volume of the nitric solution = 0.150 L
Moles of nitric acid = n
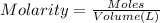
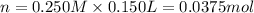
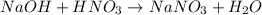
According to reaction, 1 mole of nitric acid recats with 1 mole of NaOH, then 0.0375 moles of nitric acid will react with :
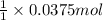 of NaOH
of NaOH
Moles of NaOH left unreacted in the solution =
= 0.375 mol - 0.0375 mol = 0.3375 mol
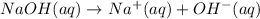
1 mole of sodium hydroxide gives 1 mol of sodium ions and 1 mole of hydroxide ions.
Then 0.3375 moles of NaOH will give :
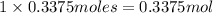 of hydroxide ion
of hydroxide ion
The molarity of hydroxide ion in solution ;
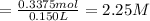
2.25 M is the final concentration of hydroxide ions ions in the solution after the reaction has gone to completion.