Answer : The unknown gas is, NO
Solution :
According to the Graham's law, the rate of effusion of gas is inversely proportional to the square root of the molar mass of gas.
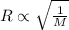
or,
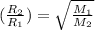 ..........(1)
..........(1)
where,
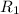 = rate of effusion of unknown gas = 31.50 mL/min
= rate of effusion of unknown gas = 31.50 mL/min
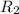 = rate of effusion of
= rate of effusion of
 gas = 30.50 mL/min
gas = 30.50 mL/min
 = molar mass of unknown gas = ?
= molar mass of unknown gas = ?
 = molar mass of
= molar mass of
 gas = 32 g/mole
gas = 32 g/mole
Now put all the given values in the above formula 1, we get:
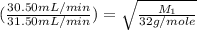
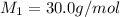
From the this we conclude that the unknown gas is, NO that has 30.0 g/mol molar mass.
Hence, the unknown gas is, NO