Answer:
The temperature at which the glass vessel shatter is = 341.33°c
Step-by-step explanation:
Initial temperature
 = 18 °c = 291 K
= 18 °c = 291 K
Initial Pressure
 = 0.9 atm
= 0.9 atm
Final pressure
 = 1.9 atm
= 1.9 atm
From ideal gas equation
P V = m R T
Here volume & mass & gas constant is constant so Pressure is directly proportional to the temperature.
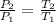
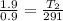
 = 613.33 K = 341.33°c
= 613.33 K = 341.33°c
Therefore the temperature at which the glass vessel shatter is = 341.33°c