Answer:
T=183.21K
Step-by-step explanation:
We have to take into account that the system is a ideal gas. Hence, we have the expression

where P is the pressure, V is the volume, n is the number of moles, T is the temperature and R is the ideal gas constant.
Thus, it is necessary to calculate n and V
V is the volume of a sphere
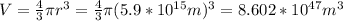
V=8.86*10^{50}L
and for n
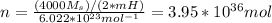
Hence, we have (1 Pa = 9.85*10^{-9}atm)
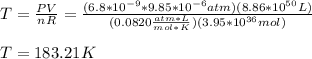
hope this helps!!