Answer:
The velocity of the photo electron is
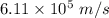 .
.
Step-by-step explanation:
Given that,
Supplied energy,
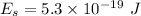
Minimum energy of the electron to escape from the metal,
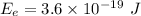
We need to find the velocity of the photo electron. The energy supplied by the photon is equal to the sum of minimum escape energy and the kinetic energy of the escaping electron. So,
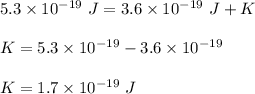
The formula of kinetic energy is given by :
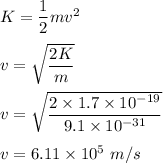
So, the velocity of the photo electron is
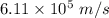 .
.