Answer:
The maximum kinetic energy of electron is = 2.93 ×
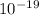 Joule
Joule
Step-by-step explanation:
We know that total energy
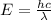 ------------ (1)
------------ (1)
Here h = plank's constant = 6.62 ×
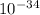 J s
J s
c = speed of light = 3 ×
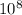
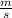
 = 261 nm = 261 ×
= 261 nm = 261 ×
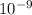 m
m
Put all these values in equation (1) we get
E = 7.6 ×
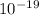 J
J
We know that
Total energy = Energy to remove an electron + K.E of electron
Energy to remove an electron =
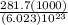
Energy to remove an electron = 4.67 ×
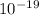 J
J
K.E of electron = Total energy - Energy to remove an electron
K.E of electron = 7.6 ×
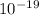 - 4.67 ×
- 4.67 ×
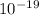
K.E of electron = 2.93 ×
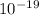 Joule
Joule
Therefore the maximum kinetic energy of electron is = 2.93 ×
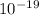 Joule
Joule