Answer: increase
Step-by-step explanation:
Henry's law states that the amount of gas dissolved or molar solubility of gas is directly proportional to the partial pressure of the liquid.
As soda contains carbon dioxide dissolved in water.
The equation given by Henry's law is:
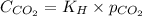
where,
 = solubility of carbon dioxide in water
= solubility of carbon dioxide in water
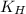 = Henry's constant
= Henry's constant
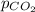 = partial pressure of carbon dioxide
= partial pressure of carbon dioxide
Thus on increasing the pressure, the solubility of carbon dioxide also increase.