Answer:
![[Pb^(2+)]=3.9 * 10^(-2)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/sd5nc9hn78hd80g39ad3yrt262shezdr2d.png)
this is the concentration required to initiate precipitation
Step-by-step explanation:
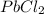 ⇄
⇄
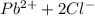
Precipitation starts when ionic product is greater than solubility product.
Ip>Ksp
Precipitation starts only when solution is supersaturated because solution become supersaturated then it does not stay in this form and precipitation starts itself only solution become saturated.
This usually happens when two solutions containing separate sources of cation and anion are mixed together and here also we are mixing lead (||)nitrate solution(source of lead(||)) into the Cl- solution.
![Ip=[Pb^(2)][2Cl^-]^2=Ksp](https://img.qammunity.org/2021/formulas/chemistry/high-school/tf26ov7l4qx7qnb929q589ehrgt52daerm.png)
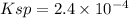
lets solubility=S
![[Pb^(2+)] = S](https://img.qammunity.org/2021/formulas/chemistry/high-school/vehk2igbkwqz21e3xsrxgziena88orlewg.png)
![[Cl^-]=2S](https://img.qammunity.org/2021/formulas/chemistry/high-school/cymmy09q2q65dr3t0k3g7rurckvck9ldpr.png)
![Ksp=[Pb^(2+)]* [Cl^-]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/rzrf94y7hqdf8v1dlgp4vq95bw85ednnwt.png)
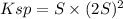
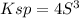
![S=\sqrt[3]{(Ksp)/(4) }](https://img.qammunity.org/2021/formulas/chemistry/high-school/qq41n514scmfm7pr7op8il7pih7by4szjc.png)
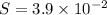
![[Pb^(2+)]=3.9 * 10^(-2)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/sd5nc9hn78hd80g39ad3yrt262shezdr2d.png) this is the concentration required to initiate precipitation
this is the concentration required to initiate precipitation