The molarity of KOH is 0.98 M.
Step-by-step explanation:
KOH + HBr →KBr + H₂O
As per the above reaction, equal moles of KBr and HBr reacts to form 1 mole of KBr and 1 mole of water. We have to find the molarity of KOH by using the law of Volumetric analysis using the equation as,
V1M1 = V2M2
Here V1 and M1 are the volume and molarity of HBr.
V2 and M2 are the volume and molarity of KOH .
We can find the molarity of KOH as,
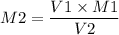
Plugin the values as,
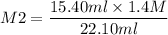
= 0.98 M
So the molarity of KOH is 0.98 M.