Answer:
(a) solubility in pure water =9.11 x 10⁻⁹ (M)
(b) solubility in 0.08 (M) NaI = 1.04 x 10⁻¹⁵ (M)
Step-by-step explanation:
(1)
AgI (s) + H₂O ⇄ Ag⁺(aq) + I⁻ (aq)
s s
Solubility product of AgI
Ksp = [Ag⁺] [I⁻]
⇒Ksp = s²
⇒s
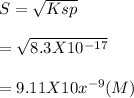
solubility in pure water
(2)
Ksp = [Ag⁺] [I⁻]
⇒ 8.3 X 10⁻¹⁷ = S x (0.08+S)
and assuming S is small relative to 0.08, due to common ion effect (I⁻) solubility of salt decreases
S + 0.08 = 0.08
⇒S X 0.08 = 8.3 X 10⁻¹⁷
⇒S = 1.04 X 10⁻¹⁵ (M) solubility in 0.08 (M) NaI