Answer:
The average kinetic energy of a gas molecule is 8.49 × 10⁻²¹ J
Step-by-step explanation:
According to Kinetic Molecular Theory, the average kinetic energy of gas molecules is a function only of temperature and is given by the equation:
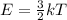
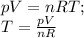
Substituting the value of T in the kinetic energy equation:
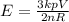
Where p is the pressure of the gas = 9 atm = 9 × 101325 pa
k is the Boltzmann’s constant = 1.38066 × 10⁻²³ J/K,
V is the volume of the gas = 7.1 L = 7.1 × 10⁻³ m³
n is the number of moles of the gas = 1.9 mol
R is the universal gas constant = 8.31451 J/K · mol.
E is the average kinetic energy of a gas molecule
Substituting values:
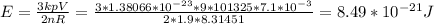
The average kinetic energy of a gas molecule is 8.49 × 10⁻²¹ J