Answer:
The limiting reactant is the 6.279 g of
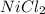
Step-by-step explanation:
We have to start with the reaction between sodium carbonate (
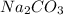 ) and the Nickel (II) Chloride (
) and the Nickel (II) Chloride (
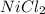 ), so:
), so:

We will have a double replacement reaction. Now we have to balance the reaction, so:

The next step is the calculation of the moles for each reactive. For
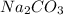 we have use the molarity equation:
we have use the molarity equation:
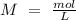
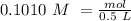
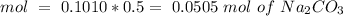
For the calculation of moles of
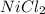 we have to use the molar mass of the compound (129.59 g/mol):
we have to use the molar mass of the compound (129.59 g/mol):
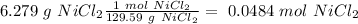
The next step is the division of each mole value by the coefficient of each reactive of the balance reaction. In this case we have "1" for each reactive, so:
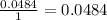
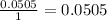
The final step is to choose the smallest value. In this case is the value that correspond to
 . Therefore
. Therefore
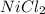 is the limiting reactive.
is the limiting reactive.