Answer:
composition of alpha phase is 33% B
Step-by-step explanation:
given data
total composition Co = 58%
β phase Cb = 83 %
mass fractions = 0.5
solution
we will use here lever rule that is
Wα = Wβ ................1
and
Wα = Wβ = 0.5
so here we will take one side i.e left
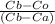 = 0.5
= 0.5
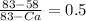
Ca = 33
so that composition of alpha phase is 33% B