This is an incomplete question, here is a complete question.
A titration reached the equivalence point when 17.0 mL of 0.211 M H₂SO₄ (aq) was added to 16.3 mL of NaOH (aq) of unknown concentration. What is the concentration (M) of this unknown NaOH solution?
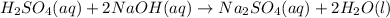
Answer : The concentration (M) of this unknown NaOH solution is, 0.440 M
Explanation :
To calculate the concentration of unknown NaOH solution, we use the equation given by neutralization reaction:
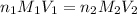
where,
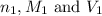 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is

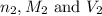 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.
We are given:
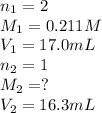
Now put all the given values in above equation, we get:
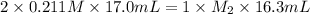
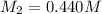
Therefore, the concentration (M) of this unknown NaOH solution is, 0.440 M