Answer: The following statements are true:
- Lithium is least easily oxidized of the metals listed on the activity series and therefore it is least likely to give electrons to metal cations
- Lithium will only react with metal ions that require the addition of one electron because of the fact that lithium only gives up one electron
Step-by-step explanation:
When a neutral atom loses an electron then it forms a positive ion due to the decrease in number of electrons. This positive ion is also known as cation.
For example, atomic number of lithium is 3 and its electronic configuration is
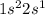 . It will attain stability by losing an electron.
. It will attain stability by losing an electron.
Whereas when a neutral atom tends to gain electrons then it acquires a negative change and hence leads to the formation of a negative ion. This negative ion is also known as anion.
As the atomic size of lithium is very small as compared to other elements of its group. So, it is difficult to extract an electron from lithium atom hence, it is not easily oxidized.
Therefore, we can conclude that the following statements are true:
- Lithium is least easily oxidized of the metals listed on the activity series and therefore it is least likely to give electrons to metal cations.
- Lithium will only react with metal ions that require the addition of one electron because of the fact that lithium only gives up one electron.