Answer:
Therefore
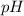 of the buffer solution is 6.96.
of the buffer solution is 6.96.
Step-by-step explanation:
The Henderson- Hasselbalch Equation:
![pH= pK_a+log ([salt])/([acid])](https://img.qammunity.org/2021/formulas/chemistry/college/ngbvkpot8xqic8yr0qfd8s8js1vzu1zjlt.png) .
.
The acid will be H₂PO₄⁻ and the salt of the acid will be HPO₄²⁻.
Given the molar mass of NaH₂PO₄ is 120.0 g/mol and the molar mass of Na₂HPO₄ is 142.0g/mol.
The number of mole of 12 gram of NaH₂PO₄ is
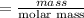
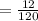
=0.1 mole
The number of mole of 8 gram of Na₂HPO₄ is
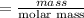
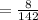
=0.056 mole.
Concentration of [H₂PO₄⁻ ] is
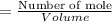
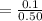
=0.2 M
Concentration of [HPO₄²⁻] is
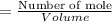
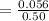
=0.112 M
Therefore ,
![pH= pK_a+log ([salt])/([acid])](https://img.qammunity.org/2021/formulas/chemistry/college/ngbvkpot8xqic8yr0qfd8s8js1vzu1zjlt.png)
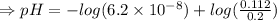
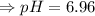
Therefore
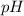 of the buffer solution is 6.96.
of the buffer solution is 6.96.