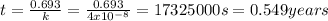Answer:
This exercise is incomplete, missing the value of k (decay constant), which is equal to 4x10⁻⁸s⁻¹ at 25°C
The answer is 0.549 years
Step-by-step explanation:
Given:
first order of reaction
k = 4x10⁻⁸s⁻¹
For a first order of reaction, the half-life time for the degradation of DDT is equal to: