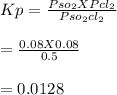Answer:
The value of Kp for the system = 0.0128 atm
Step-by-step explanation:
Let, the gaseous substance is SO₂Cl₂
SO₂Cl₂ ⇄ SO₂ + Cl₂
Initially x 0 0
At equilibrium (x - 14.5%x) 14.5%x 14.5%x
= 85.5 % x
Total moles of gases at equilibrium
= 85.5 % x + 14.5%x + 14.5%x
= 114.5 % x
Partial pressure of SO₂ (
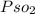 ) =
) =
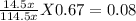
Partial pressure of Cl₂
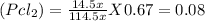
Partial pressure of SO₂Cl₂
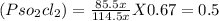
So,