Answer:
Step-by-step explanation:
1. Balanced chemical equation
The chemical equation that represents the synthesis reaction of water from hydrogen and oxygen is:
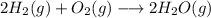
2. Mole ratios
The coefficients of the balanced chemical reaction represent the mole ratios in which the reactants combine and form the products.
In this case that is:
- 2 moles H₂ : 1 mole O₂ : 2 moles H₂O
- 2 : 1 : 2
3. Volumetric ratios
When the reaction proceeds at constant pressure and temperature, the law of ideal gases implies that the mole ratios are equal to the volumetric ratios.
Then, you can also state:
- 2 liter H₂ : 1 liter O₂ : 2 liter H₂O
Thus, the number of liters of gaseus water produced is equal to the number of liters o fhydrogen gas used, and you conclude that, when you start with 1.7 liter of hydrogen, the yield of water is 1.7 liter.
- 1.7 liter of H₂O ← answer