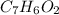Answer:
Step-by-step explanation:
We have to assume that we have 100 g of the unknow as first step. With this in mind we can calculate the grams of C, H and O in the sample.
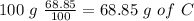
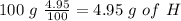
For the calculation of O we have to know the percentage of O therefore we can add the percentages of H and C and then do a substraction from 100 %, so:
 100-(68.85+4.95)=26.2 %
100-(68.85+4.95)=26.2 %
With this value we can calculate the amount of O in grams in the sample:
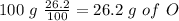
The next step is the calculation of moles for each atom, for this we have to know the atomic mass of each atom ( C: 12 g/mol; H 1 g/mol; O 16 g/mol):
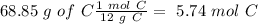
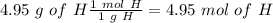
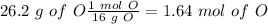
Now we have to divide by the smallest value, so:
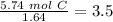
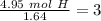
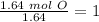
We obtain an decimal number for C, therefore we have to multiply all by "2", so:
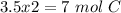
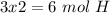
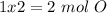
Therefore the formula would be: