Answer : The molecular weight of the unknown gas is, 57.0 amu
Explanation :
According to the Graham's law, the rate of effusion of gas is inversely proportional to the square root of the molar mass of gas.
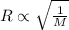
And the relation between the rate of effusion and volume is :
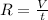
From this we conclude that,
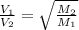
where,
 = rate of effusion of unknown gas = 11.50 mL
= rate of effusion of unknown gas = 11.50 mL
 = rate of effusion of argon gas = 9.63 mL
= rate of effusion of argon gas = 9.63 mL
 = molar mass of unknown gas
= molar mass of unknown gas
 = molar mass of argon gas = 40 amu
= molar mass of argon gas = 40 amu
Now put all the given values in the above formula, we get:
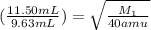
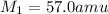
Therefore, the molecular weight of the unknown gas is, 57.0 amu