Answer:
The mass of
 in the container is 2.074 gram
in the container is 2.074 gram
Step-by-step explanation:
Given:
Volume of
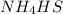
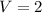 lit
lit
Equilibrium constant
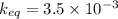
The reaction in which
 is produced
is produced
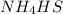 ⇄
⇄
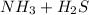
Here equal moles of
 and
and
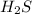 is formed.
is formed.
From the formula of equilibrium constant,
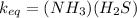
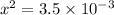
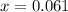 M
M
Above value shows,
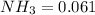
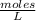
So in 2 L no. moles of
 =
=
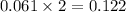 moles.
moles.
So mass of 0.122 mole of
 is =
is =
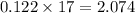 g
g
Therefore, the mass of
 in the container is 2.074 gram
in the container is 2.074 gram