Answer:
Concentration of acetic acid=2.3mol/L
Step-by-step explanation:
Write the balanced chemical reacion:
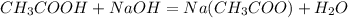
N1 and N2 normality of CH3COOH and NaOH respectively;
V1 and V2 volume of CH3COOH and NaOH respectively and they are taken upto end point of titration;
we have given molarity but we need normality;
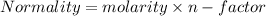
but in case of NaOH and CH3COOH n-factor is 1 for each.
hence
normality=molarity;
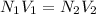
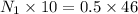
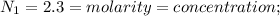
Concentration of acetic acid=2.3mol/L