Answer:
The amount of final volume when mass of the system is constant =
0.526 L
Step-by-step explanation:
Given data
 = 500 mL = 0.5 L
= 500 mL = 0.5 L
 = 740 torr
= 740 torr
 = 25 ° c = 298 K
= 25 ° c = 298 K
 = 50 ° c = 323 K
= 50 ° c = 323 K
 = 760 torr
= 760 torr
From ideal gas equation we get
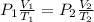
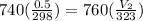
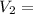 0.526 L = 526 ml
0.526 L = 526 ml
This is the amount of final volume when mass of the system is constant.