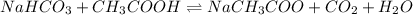Answer:
This is an example of acid-base reaction.
Step-by-step explanation:
Sodium bicarbonate is an weak base. Vinegar (acetic acid) is an weak acid.
So, mixing sodium bicarbonate with vinegar would result an acid-base reaction.
In this acid-base reaction, gaseous carbon dioxide is formed along with heat evolution as acid-base neutralization is exothermic in nature.
Hence, a volcanic eruption occurs.
Balanced reaction: