Answer: There are 7 alpha-particle emissions and 4 beta-particle emissions involved in this series
Step-by-step explanation:
Alpha Decay: In this process, a heavier nuclei decays into lighter nuclei by releasing alpha particle. The mass number is reduced by 4 units and atomic number is reduced by 2 units.
Beta Decay : It is a type of decay process, in which a proton gets converted to neutron and an electron. This is also known as -decay. In this the mass number remains same but the atomic number is increased by 1.
In radioactive decay the sum of atomic number or mass number of reactants must be equal to the sum of atomic number or mass number of products .
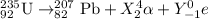
Thus for mass number : 235 = 207+4X
4X= 28
X = 7
Thus for atomic number : 92 = 82+2X-Y
2X- Y = 10
2(7) - Y= 10
14-10 = Y
Y= 4
Thus there are 7 alpha-particle emissions and 4 beta-particle emissions involved in this series