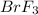Answer: The empirical formula is
Step-by-step explanation:
If percentage are given then we are taking total mass is 100 grams.
So, the mass of each element is equal to the percentage given.
Mass of Br= 58.37 g
Mass of F = (100-58.37) = 41.63 g
Step 1 : convert given masses into moles.
Moles of Br=
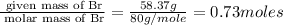
Moles of F =
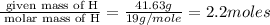
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For Br =
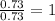
For F =
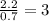
The ratio of Br: F= 1 : 3
Hence the empirical formula is