Answer:
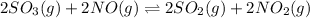
Step-by-step explanation:
Relation of
 with
with
 is given by the formula:
is given by the formula:
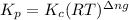
where,
 = equilibrium constant in terms of partial pressure
= equilibrium constant in terms of partial pressure
 = equilibrium constant in terms of concentration
= equilibrium constant in terms of concentration
R = Gas constant
T = temperature
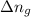 = change in number of moles of gas particles =
= change in number of moles of gas particles =

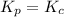 when
when
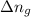 = 0
= 0
a)
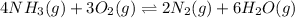
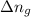 = change in number of moles of gas particles =
= change in number of moles of gas particles =
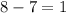
b)
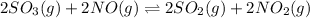
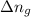 = change in number of moles of gas particles =
= change in number of moles of gas particles =
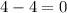
c)
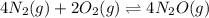
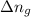 = change in number of moles of gas particles =
= change in number of moles of gas particles =
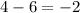
d)
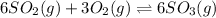
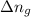 = change in number of moles of gas particles =
= change in number of moles of gas particles =
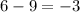
Thus for reaction ,
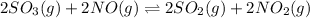 ,
,
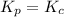 as
as
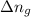 = 0
= 0