Answer :
AgBr should precipitate first.
The concentration of
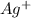 when CuBr just begins to precipitate is,
when CuBr just begins to precipitate is,
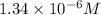
Percent of
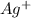 remains is, 0.0018 %
remains is, 0.0018 %
Explanation :
 for CuBr is
for CuBr is
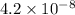
 for AgBr is
for AgBr is
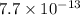
As we know that these two salts would both dissociate in the same way. So, we can say that as the Ksp value of AgBr has a smaller than CuBr then AgBr should precipitate first.
Now we have to calculate the concentration of bromide ion.
The solubility equilibrium reaction will be:
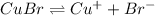
The expression for solubility constant for this reaction will be,
![K_(sp)=[Cu^+][Br^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/csvsu4ut0bgfiy7dw4spjow87eovwhpfrg.png)
![4.2* 10^(-8)=0.073* [Br^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/150gd4469otwmsax4cxfq699ca34i2q1q7.png)
![[Br^-]=5.75* 10^(-7)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/owujmppm6nzt6ei1v47v6i2d0d2vgk32zt.png)
Now we have to calculate the concentration of silver ion.
The solubility equilibrium reaction will be:
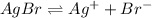
The expression for solubility constant for this reaction will be,
![K_(sp)=[Ag^+][Br^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/lgkpvfvk8twa5of6wlh1nsl1lwyoyq4xdy.png)
![7.7* 10^(-13)=[Ag^+]* 5.75* 10^(-7)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/5nyrvdgvzgr5ivbz4kkcgbultogufugsvv.png)
![[Ag^+]=1.34* 10^(-6)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/impwbpjnogsqq3fszeswj5vxmk7mfoo284.png)
Now we have to calculate the percent of
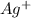 remains in solution at this point.
remains in solution at this point.
Percent of
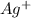 remains =
remains =
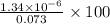
Percent of
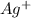 remains = 0.0018 %
remains = 0.0018 %