Answer:
Step-by-step explanation:
When the volume and temperature of a gas are unchanged, the pressure is directly related to the number of molecules, this is:
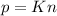
Or:
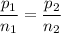
Thus:
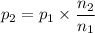
For the reaction 3A(g) → 2B(g), n₁ = 3 and n₂ = 2.
Then:
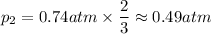
As you see, the pressure decreases, because the number of molecules decreases.