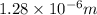Answer : The wavelength of the photon emitted is,
Explanation :
Using Rydberg's Equation:
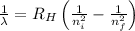
Where,
 = Wavelength of radiation
= Wavelength of radiation
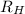 = Rydberg's Constant = 1.096776 × 10⁷ m⁻¹
= Rydberg's Constant = 1.096776 × 10⁷ m⁻¹
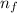 = Higher energy level = 5
= Higher energy level = 5
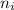 = Lower energy level = 3
= Lower energy level = 3
Now put all the given values, in above equation, we get:
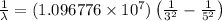
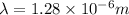
Therefore, the wavelength of the photon emitted is,