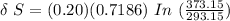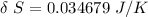Answer:
Step-by-step explanation:
The formula for calculating the change in entropy of a process can be expressed as:
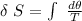
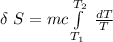
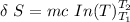
![\delta \ S = mc \ [In \ (T_2) - In \ ({T_1})]](https://img.qammunity.org/2021/formulas/physics/college/txckjggsl7p8ngetu6juwh4ojag5fy6qk3.png)
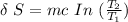
Given that:
mass m = 0.20 kg
specific heat constant c = 0.7186J/ Kg K
 = 100° C = ( 100 + 273.15) = 373.15 K
= 100° C = ( 100 + 273.15) = 373.15 K
 = 20° C = ( 20 + 273.15) = 293.15 K
= 20° C = ( 20 + 273.15) = 293.15 K
Replacing our values into above equation; we have :